Case Report
Lapatinib and capecitabine in a heavily-pretreated patient with HER2-positive metastatic breast cancer
1 Consultant Physician of Internal Medicine and Medical Oncology, Ambulatory Medicine Hamad, Medical Corporation Doha-State of Qatar
2 Pre-college Volunteer Student at London Central Secondary School, Applicant for Medical School at Weill Cornell-Qatar (WCM-Q),
Address correspondence to:
M. S. Ahmed
Consultant Physician of Internal Medicine and Medical Oncology/Ambulatory Medicine, Hamad Medical Corporation, P.O. Box - 3050,
Doha-State of Qatar
Access full text article on other devices

Access PDF of article on other devices

Article ID: 100007C01MA2018
doi: 10.5348/100007C01MA2018CR
How to cite this article
Ahmed MS, Sadiq YM. Lapatinib and capecitabine in a heavily-pretreated patient with HER2-positive metastatic breast cancer. Edorium J Cancer 2018;4:100007C01MA2018.ABSTRACT
Introduction: Lapatinib is a dual tyrosine kinase inhibitor of both epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor type 2 (HER2 or ErbB2). It is approved in combination with capecitabine for the treatment of HER2-receptor positive advanced or metastatic breast cancer (MBC) that has progressed on trastuzumab therapy.
Case Report: A 49-year-old lady, who was previously treated for right sided breast cancer, presented to the National Centre of Cancer Care and Research in Qatar with local recurrence and multiple metastases to the liver. Her disease progressed within one year of diagnosis despite lumpectomy and adjuvant chemotherapy. For unknown reason, she was not offered adjuvant radiotherapy. HER2-receptor analysis was not available back in her home country and was not accessible for the patient either. The recurrent disease treated with modified radical mastectomy. Surgery was followed by two cycles of Carboplatin and Vinorelbine at first then shifted to a combination of Paclitaxel and Trastuzumab when Her-2 receptor analysis came as 90% positive, and followed by maintenance monotherapy of Trastuzumab for eight cycles. The patient progressed with brain metastases that were treated with whole brain irradiation followed by Lapatinib and Capecitabine combination therapy for a period of 12 months.
Conclusion: Based on this case, medical oncologists would realize the effectivity of lapatinib plus Capecitabine to treat patients with metastatic breast cancer, mainly those with brain metastases, but also to provide stability of other soft tissue metastases. Lapatinib based therapy achieved an excellent control of the brain metastases despite interruption of treatment for two months; a response that is translated into prolonged overall survival and or time to progression.
INTRODUCTION
Lapatinib is a dual tyrosine kinase inhibitor of both epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor type 2 (HER2 or ErbB2) [1],[2],[3]. It is approved in combination with capecitabine for the treatment of HER2- receptor positive advanced or metastatic breast cancer (MBC) that has progressed on trastuzumab therapy [4].
CASE REPORT
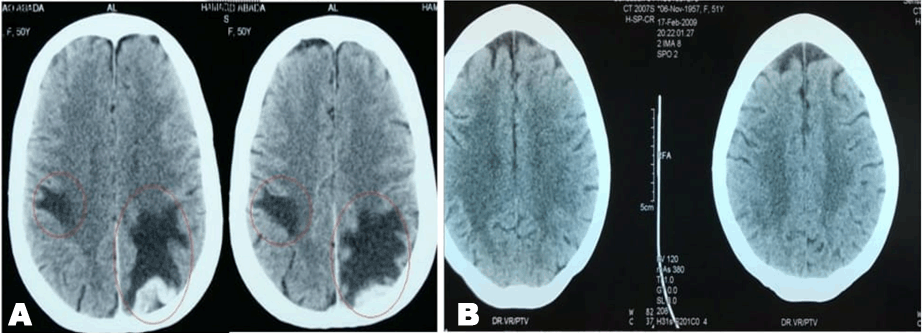
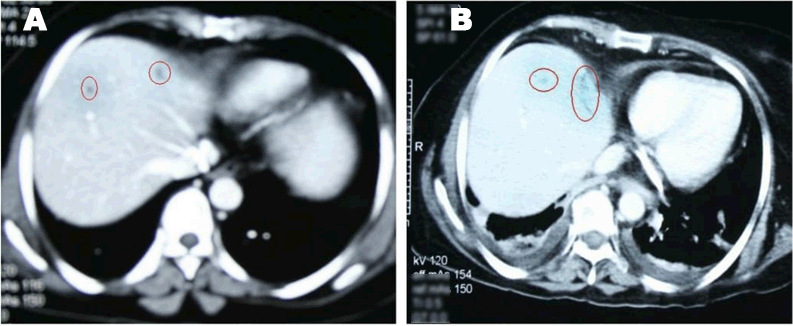
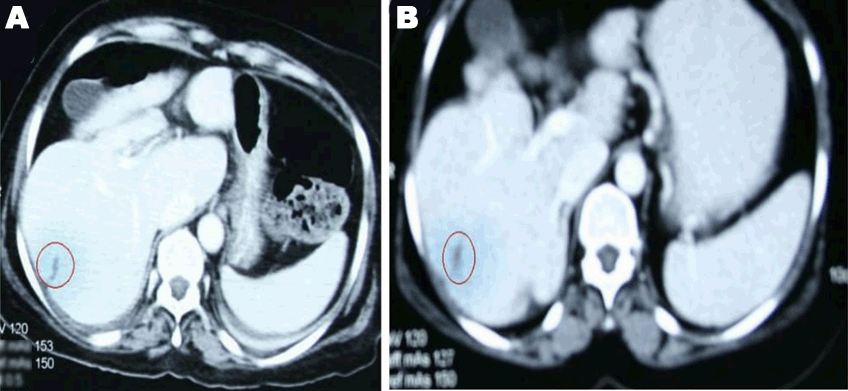
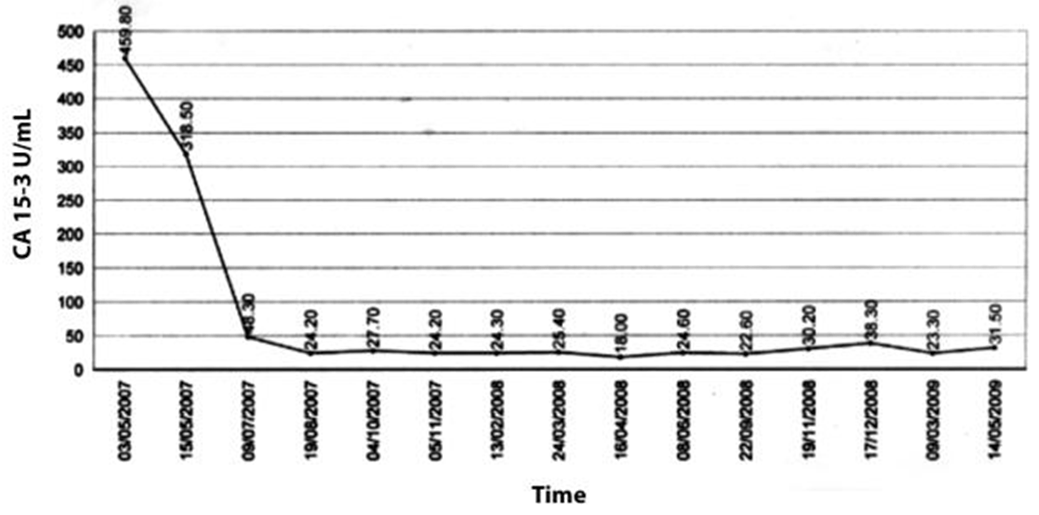
A 49-year-old female was diagnosed with infiltrating ductal carcinoma of the right breast in May 2006. The patient underwent lumpectomy and histopathology showed T2N1M0 with ER-ve, PR-ve but no HER2- receptor analysis was available for the patient. Surgery was followed by six cycles of adjuvant chemotherapy with AC (doxorubicin and cyclophosphamide). She received no radiotherapy at that time. In February 2007, the patient presented to our center with right breast lump associated with palpable right axillary mass that were radiologically and pathologically confirmed to be a local recurrence with liver metastases. She underwent radical mastectomy with axillary clearance. The tumor measured 3x2 cm, and 13 out of 13 lymph nodes were involved. Histopathology showed infiltrating ductal carcinoma, grade 2, T2N3M1, ER-, PR-, HER2- testing was sent abroad, so the patient began carboplatin and vinorelbine. In May 2007, a CT scan showed progressive disease with dorsal spine metastasis in addition to the liver metastasis and HER2- was received as 90% positive; accordingly, therapy was changed to trastuzumab, paclitaxel, and zoledronic acid. The patient showed clinical improvement with a decreasing CA 15-3 and stable disease by CT scan. She completed 8 cycles of chemotherapy and continued on maintenance trastuzumab therapy. After single-agent trastuzumab for 8 more cycles, she developed right-sided weakness with generalized tonic-clonic seizures. She was confirmed to have brain metastasis and was given a course of palliative whole brain irradiation, which completed in April 2008. She also started lapatinib 1250 mg/day and capecitabine 2000 mg/m2/day in March 2008. By mid-October 2008, lapatinib treatment was interrupted due to its unavailability, and the patient was kept on capecitabine with resumption of trastuzumab to sustain the clinical and the biochemical response achieved with lapatinib (Graph 1). In December 2008, lapatinib was resumed with capecitabine, trastuzumab, and zoledronic acid. While on lapatinib, CT scan re-assessment demonstrated dis-appearance of the brain metastases (Figure 1) and stable metastatic lesions in the liver (Figures 2 & 3).
In April 2009, the patient progressed with recurrence of multiple brain metastases. She became severely weak, aphasic, and dysphagic. The patient was then admitted for palliative supportive care. After improvement of her general condition, she was to start bevacizumab and topotecan, which was unavailable at that time; meanwhile the patient was kept on bevacizumab and vinorelbine. In June 2009, the patient changed to bevacizumab and topotecan until she died in October 2009.
DISCUSSION
Most studies in the literature show a time to progression (TTP) for patients with MBC treated with lapatinib of approximately 6 to 8 months. In a previous study, the median TTP with lapatinib and capecitabine was 8.4 months compared to single-agent capecitabine, which showed a median TTP of 4.4 months [5]. In another phase III randomized comparison in women with MBC that had progressed on trastuzumab, the median TTP in the lapatinib and capecitabine arm was 27.1 weeks compared to the single-agent arm of capecitabine with a TTP of 18.6 weeks [6].
This case is unique because lapatinib was given for one year with very good tolerability and almost no side effects [7]. Moreover, during treatment brain disease control and visceral “hepatic” disease stability confirmed radiologically (Figures 1, 2, 3) in addition to the reduction and later on plateauing of CA 15-3 levels (Graph 1). There was a 2-month period when lapatinib was unavailable and treatment was interrupted. If lapatinib was continuously available, the outcome might have been positively different. Beside if treatment with lapatinib started earlier than or as combined with trastuzumab, when patient was first discovered to have metastatic disease, the disease course might have been a better one [8].
In this case of aggressive heavily-pretreated MBC, the use of Lapatinib for almost 14 months (including two months of interruption) was associated with favorable tolerability and disease stability. Continuity of treatment is a pre-requisite, especially with such aggressive and rapidly progressive cancers. An upfront modality of anti-HER-2neu combination were under investigation that time [9], although it could have been an option; as with the recent hope of Trastuzumab and Pertuzumab combination [10]. This alerted us to explore trial or experimental agents with clients who are still in a good performance status to proceed with chemo and or targeted therapies. Finally, genetic expression profiling [11],[12] and biological subtyping [13] would have been a helpful option for this patient but likely the availability of samples and facilities to process them was an issue to hinder it.
CONCLUSION
With all current advances in the treatment of breast cancer, the latter still ranked second among other malignant disorders as a cause of mortality and morbidity. Large scale phase-3 and phase-4 trials are ongoing to search for an effective, safe, and convenient therapies to tackle this debilitating cancer. Lapatinib plus capecitabine have been proved as an excellent option to treat patients progressed with brainmetastases after they failed other chemotherapies, hormonal therapies, and Radiotherapy. The aforementioned regimen has confirmed a response in term of time to progression and overall survival. This case presented an evidence of response to lapatinib based combination even after interruption of treatment to the extent of providing stable disease for other soft tissue metastases. Considering its safety profile and convenient route of administration, further studies have to explore the efficacy of lapatinib in combination with other chemotherapy agents or Her-2 receptors’ antagonists in treating metastatic breast cancer with brain metastases or even other soft tissue metastases.
REFERENCES
1.
Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): Relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res 2004 Sep 15;64(18):6652–9. [CrossRef]
[Pubmed]

2.
Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res 2006 Feb 1;66(3):1630–9. [CrossRef]
[Pubmed]

3.
Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 2007 Apr 18;99(8):628–38. [CrossRef]
[Pubmed]

4.
Ryan Q, Ibrahim A, Cohen MH, et al. FDA drug approval summary: Lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist 2008 Oct;13(10):1114–9. [CrossRef]
[Pubmed]

5.
Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006 Dec 28;355(26):2733-43. [CrossRef]
[Pubmed]

6.
Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008 Dec;112(3):533–43. [CrossRef]
[Pubmed]

7.
Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol 2008 Jun 20;26(18):2999–3005. [CrossRef]
[Pubmed]

8.
Guarneri V, Frassoldati A, Piacentini F, et al. Preoperative chemotherapy plus lapatinib or trastuzumab or both in HER2-positive operable breast cancer (CHERLOB Trial). Clin Breast Cancer 2008 Apr;8(2):192–4. [CrossRef]
[Pubmed]

9.
Xu ZQ, Zhang Y, Li N, et al. Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017 Mar 13;7(3):e013053. [CrossRef]

10.
Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015 Feb 19;372(8):724–34. [CrossRef]
[Pubmed]

11.
van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002 Dec 19;347(25):1999–2009. [CrossRef]
[Pubmed]

12.
Sauter G, Simon R. Predictive molecular pathology. N Engl J Med 2002 Dec 19;347(25):1995–6. [CrossRef]
[Pubmed]

13.
Sihto H, Lundin J, Lundin M, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: A nationwide cohort study. Breast Cancer Res 2011 Sep 13;13(5):R87. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
M. S. Ahmed - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Y. M. Sadiq - Substantial contributions to conception and design, Acquisition of data, Analysis of data, Interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Data Availability StatementThe corresponding author is the guarantor of submission.
Consent For PublicationWritten informed consent was obtained from the patient for publication of this case report.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Competing InterestsAuthors declare no conflict of interest.
Copyright© 2018 Mohammed Sadeq Ahmed et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.